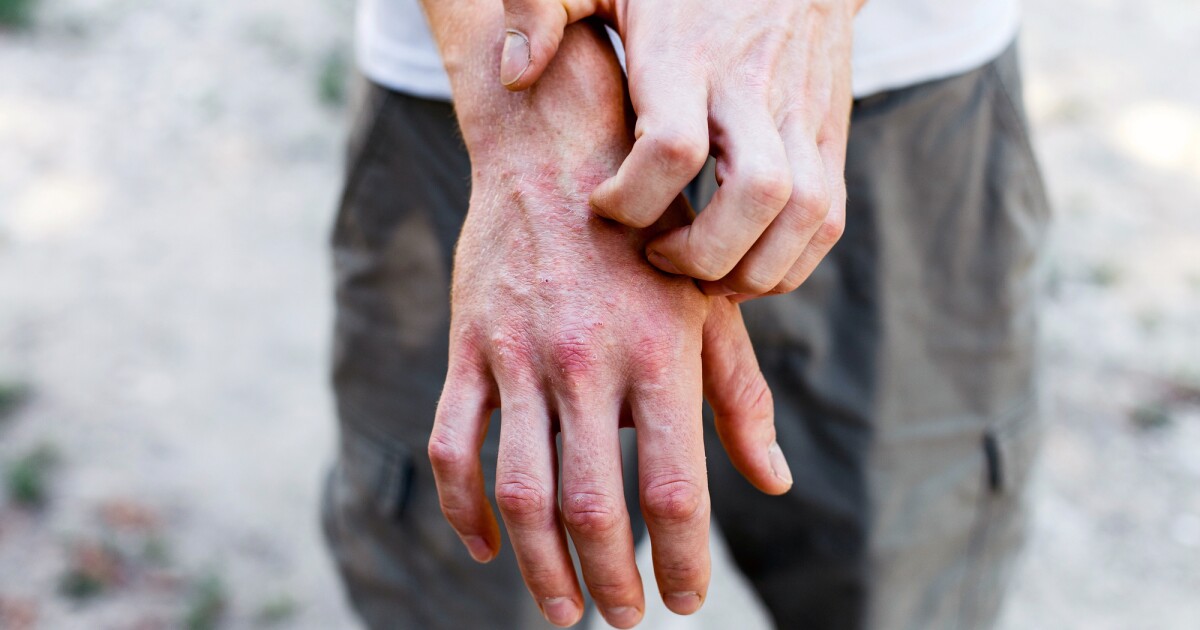
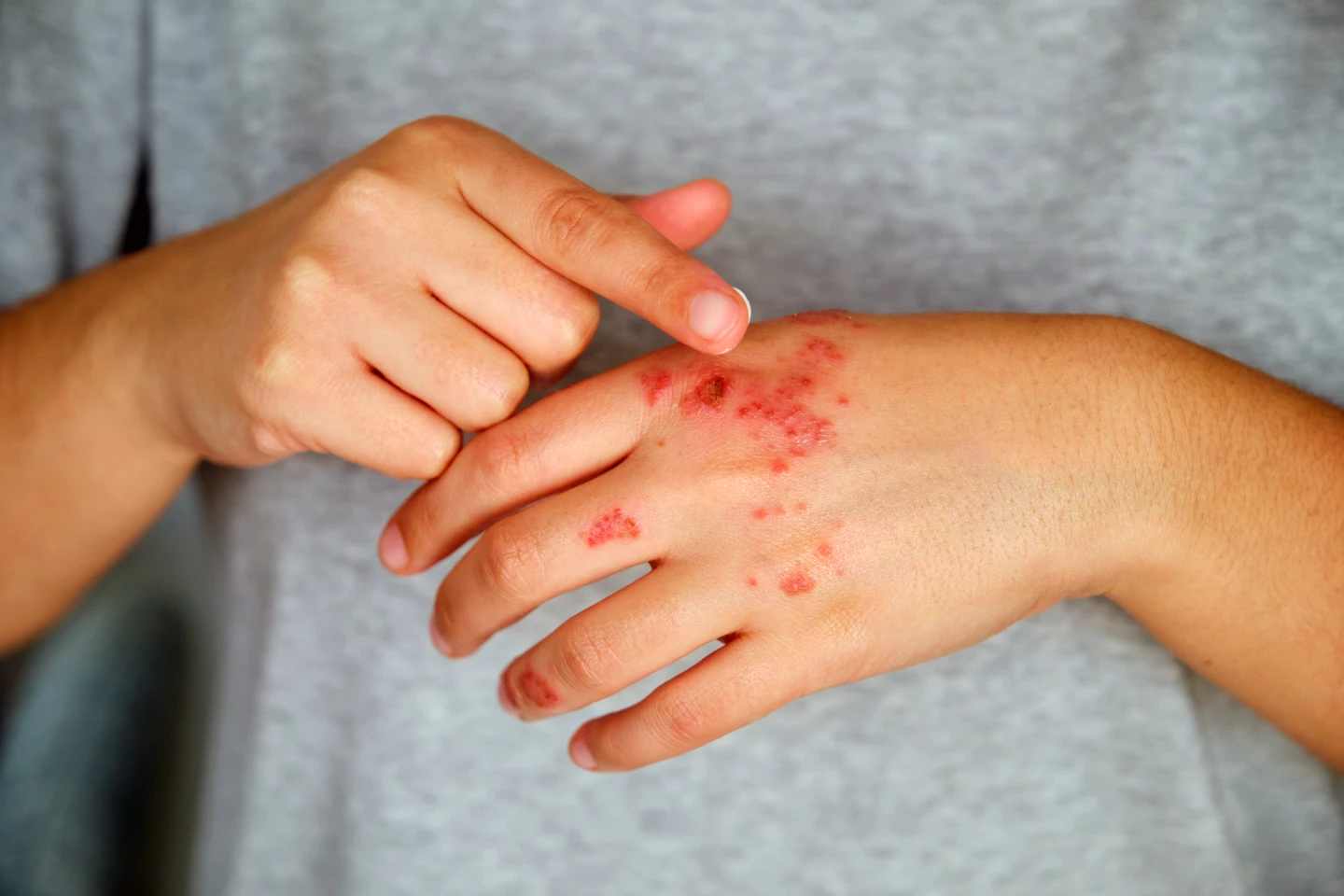
A new immune-modulating treatment has shown promising results for treating moderate to severe eczema, delivering fast itch relief and clear skin for many patients in a clinical trial, without the common side effects associated with other therapies.
For some people, the chronic skin condition atopic dermatitis, the most common and best-known type of eczema, can be challenging to treat, especially if it’s caused by something that can’t be controlled, such as genetics. Some resort to using a combination of topical ointments and creams to try to control the dry skin and itching that characterize the condition.
However, help may soon be at hand in the form of rezpegaldesleukin, an immunotherapy treatment developed by Nektar Therapeutics, which calms the symptoms of eczema from the inside out and has produced promising results in a recent clinical trial.
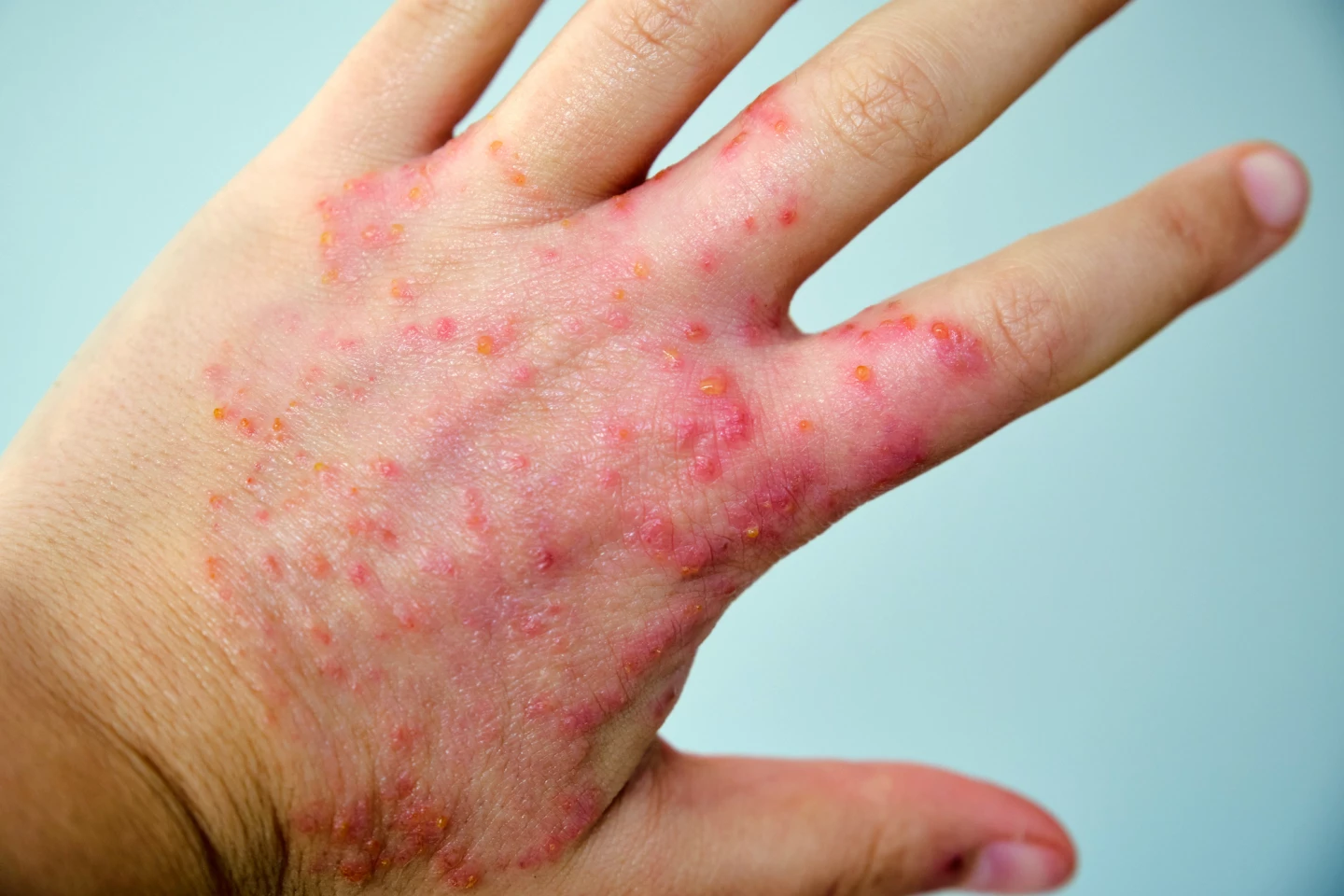
Thankfully, rezpegaldesleukin is also known by the less tongue-twisty name, Rezpeg (or NKTR-358). Rezpeg is designed to rebalance the immune system by boosting regulatory T cells (Tregs), special cells that help calm inflammation. It’s injected under the skin and targets the receptor on the surface of certain immune cells that binds to the messenger protein interleukin-2 (IL-2), a crucial regulator of the immune system. It works by increasing levels of Tregs and reducing key inflammatory markers.
The trial enrolled 393 adults and adolescents over 12 from Europe, the US, Canada, and Australia, with moderate-to-severe atopic dermatitis. They were randomly assigned to receive a placebo or one of three doses of Rezpeg: high dose (24 µg/kg every two weeks), middle dose (18 µg/kg every two weeks), or low dose (24 µg/kg every four weeks). The treatment’s effectiveness was evaluated after 16 weeks, using various tools.
The Eczema Area and Severity Index (EASI) measures the severity and extent of eczema symptoms such as redness, thickness, scratching, and skin damage across four body regions and is the gold-standard tool for tracking overall disease severity. Intensity and area scores are combined to yield a total score ranging from zero (no eczema) to 72 (most severe). The terms EASI-50, EASI-75, and EASI-90 refer to patients achieving 50%, 75%, and 90% improvement or more from their baseline score.
The Validated Investigator’s Global Assessment for Atopic Dermatitis (vIGA-AD) is a clinician rating of eczema severity on a five-point scale from zero (clear) to four (severe). The goal for a treatment is to achieve vIGA-AD 0/1, which would indicate “clear” or “almost clear” skin. The Itch NRS (Numerical Rating Scale) measures patient-reported itch severity from zero (no itch) to 10 (worst imaginable itch). A four-point or greater reduction in Itch NRS is considered clinically meaningful. BSA, or body surface area, is a measurement of skin affected by eczema, expressed as a percentage, which is helpful for quantifying how widespread the disease is.
By week 16, Rezpeg worked significantly better than placebo across all dosing groups, with the greatest improvements seen in the high- and middle-dose groups. Participants’ EASI score had improved by 61% in the high dose group, 58% in the middle dose group, and 53% in the low dose group versus a 31% improvement in the placebo group. Up to 46% of participants achieved EASI-75 compared to 17% in the placebo group. Up to 42% saw a clinically significant four-or-more point reduction in Itch NRS score versus 17% in the placebo. And, up to 26% reached the goal vIGA-AD score of clear/almost clear skin versus 8% in the placebo. The treatment showed similar effectiveness for both moderate and severe eczema.
The treatment was generally well-tolerated, with most side effects being mild or moderate. The most common side effect was injection site reactions, which occurred in 70% of patients and were mostly mild and temporary. Other mild-to-moderate side effects in Rezpeg patients included eosinophilia (a high number of eosinophils, a type of white blood cell that helps regulate inflammation) (7.8% vs. 2.7% placebo), fever (6.3%), headache (6.3%) and joint pain (5.0%). Serious adverse events were rare (around 1.6% overall) and resolved without long-term issues. Importantly, there was no increased risk of side effects such as conjunctivitis, oral ulcers and herpes infections, which are often seen with other eczema medications, so that is a positive differentiator for Rezpeg.
“These data from [the] REZOLVE-AD [trial] show a fast onset of both EASI response and itch relief within the first few doses of rezpegaldesleukin treatment, which are important metrics for physicians as they assess treatment options in atopic dermatitis,” said Jonathan Silverberg, MD, PhD, MPH, Professor of Dermatology at George Washington University School of Medicine and Health Sciences. “this shows the advantage of broad-based Treg mechanism over other immune-modulation approaches in development to treat the disease. Additionally, we don’t see any increased risk of incidence of conjunctivitis, oral herpes, or oral ulcers with this mechanism of action as we do with other mechanisms.”
In February 2025, the US FDA granted Rezpeg Fast Track designation for the treatment of adults and adolescents aged 12 and over with moderate-to-severe eczema that is not adequately controlled with topical treatments or when those treatments aren’t advisable. This will enable Nektar Therapeutics to accelerate the drug’s development due to its potential to address an unmet medical need.
Long-term results of Rezpeg from the 52-week follow-up are expected in early 2026. Nektar is currently running a related trial using rezpegaldesleukin to treat alopecia areata, an autoimmune condition that causes hair loss, with results expected later this year.
Source: Nektar Therapeutics